Since the first batch of non-vitamin mineral raw materials were included in the catalogues of health food raw materials in November 2020, the State Administration for Market Regulation, the National Health Commission and the National Administration of Traditional Chinese Medicine have released 10 raw materials included in the catalogues of health food raw materials in three batches, which are coenzyme Q10, Ganoderma lucidum spore powder, spirulina, fish oil, melatonin, soy protein isolate, whey protein, ginseng, American ginseng and Lingzhi.
In accordance with the requirements of the Administration Measures of Registration and Filing for Health Food, the health food produced by using these raw materials in China needs to be filed, and can be produced and sold after approval. The foods produced by importing these raw materials, if sold as health food and advertised for the corresponding health function, they should be registered as health food in the SAMR. Only after obtaining the registration approval can they be sold as health food and advertised for their health functions.
Antion will introduce the specific requirements for the usage, suitable people, health functions, dosage form, etc. of these raw materials.
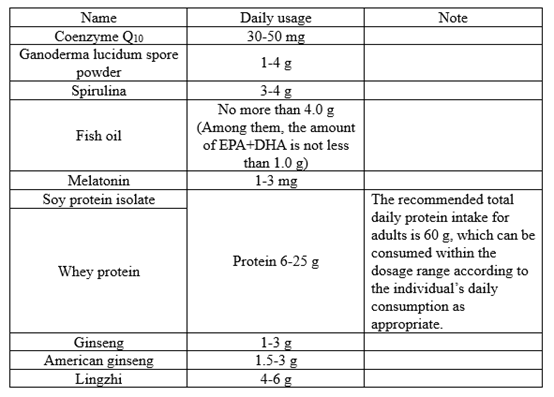
01 Requirements for usage
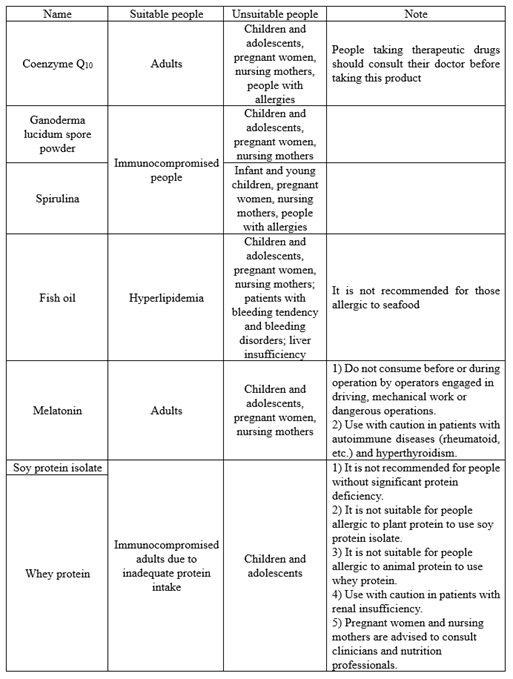
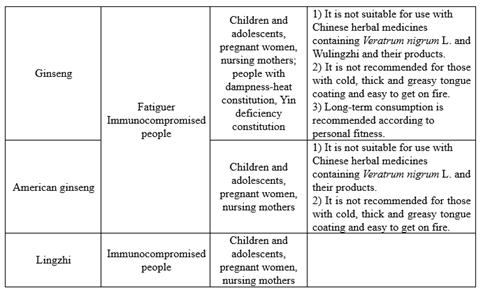
02 Suitable people
03 Health functions & dosage form
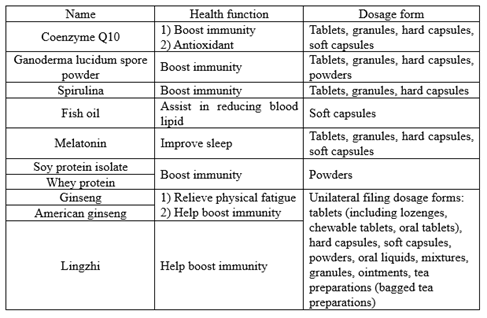
For raw materials with two health functions such as coenzyme Q10, ginseng and American ginseng, when filing, the filing person can choose one or two functions at the same time. Future product should also be marked in accordance with the type and quantity of functions filed. The health functions of ginseng and American ginseng should also correspond to their suitable people.
Source: Antion
Note: This article is compiled by Antion. Please indicate the source for reprint.