Food claims are informative statements or representations that describe the attributes, qualities, or advantages of a food or its ingredients. They serve to educate consumers about the nutritional value, health benefits, and safety aspects of food products. However, regulations and standards for food claims can vary across different countries. This article aims to provide a comparative analysis of the regulatory frameworks governing food claims in China and Canada.
Overview of food claims in China
As stipulated in GB 28050 General Rules for Nutrition Labeling of Prepackaged Foods, China allows common prepackaged food products to carry two types of food claims, namely, nutrition claims and nutrient function claims:
(1) Nutrition claims refer to the descriptions and claims of the nutritional properties of a food, such as energy value and protein content, etc. To be more specific, nutrition claim includes nutrient content claim and nutrient comparative claim:
l Nutrient content claims describe the energy value or content of a nutritional component. Dictions for nutrient content claim include "contains", "high", "low", and "no", etc. Appendix C of GB 28050 specifies the requirements and restrictive conditions for the content claims for energy and eight kinds of nutrients. Take those targeting at fat for example:
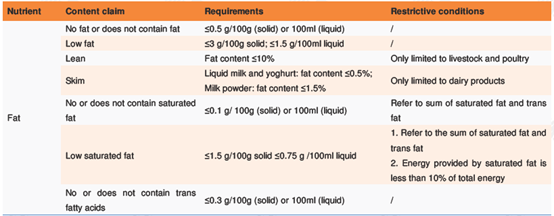
l Nutrient comparative claims are claims made after comparing the energy value or content of a nutritional component in the target food product with a well-known food in the same food type. Diction for nutrient comparative claim includes "added", "reduced", etc. Appendix C of GB 28050 specifies the requirements and conditions for comparative claims for energy and nine nutrients. For example:

(2) Nutrient function claims describe the role of a nutritional component in maintaining the growth, development and normal physiological function of the human body. Appendix D of GB 28050 lists the permitted function claims for energy and 23 kinds of nutrients. Take dietary fiber for an example, it is allowed to claim "dietary fiber helps maintain normal function of intestines" and "dietary fiber is a low energy substance".
Moreover, in China, health food, also called health supplement, is regulated as special food. Health food faces special regulations on the use of claims. As of now, China has identified a total of 24 health function claims for health food in Health Food Function Catalogue, such as "help enhance immunity". Notably, it's forbidden to use health function claims for common food products. In addition, the authorities encourage relevant enterprises to continuously research and innovate, and conditionally approve the use of new claims. As per Detailed Rules on Implementation for Technical Evaluation of New Functions and Health Food with New Functions (Trial), the new health function claims shall be classified into three major categories, namely, supplementing dietary nutrients, maintaining or improving body health, and reducing disease risk factors.
Importantly, all food products including health food shall not claim to prevent or treat diseases.
Overview of food claims in Canada
Canada allows a lot of claims for food products in various aspects, such as advertising, allergens and gluten, composition and quality, origin, production method, etc. Among them, major food claims are nutrition claims, which are optional statements on food labels that highlight specific nutrients, foods or ingredients. In the Canadian food regulatory framework, there are two types of nutrition claims:
(1) Nutrient content claims are statements that describe the amount of a nutrient in a food, such as "low in sugars", "reduced in sodium", and "excellent source of calcium". The criteria for such claims are listed in Table of Nutrient-Content Claims and What They Mean. Take the "free, no, 0, zero, without" type of claims for example: The food provides an amount of a nutrient that is so small that it likely won't have any effect on your body.
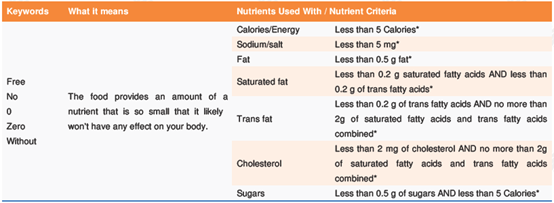
* refers to per reference amount and serving of stated size (specific amount of food listed in the Nutrition Facts table) or per 100 g if the food is a pre-packaged meal.
Depending on nutrients, nutrient content claims can be further categorized into 12 types, including: energy and calorie claims, protein claims, fat claims, saturated fatty acid claims, trans fatty acid claims, omega-3 and omega-6 polyunsaturated fatty acid claims, cholesterol claims, sodium (salt) claims, potassium claims, carbohydrate and sugars claims, dietary fibre claims, and vitamin and mineral nutrient claims.
l Comparative nutrient content claims are those that compare the nutritional properties of two or more foods. Examples of comparative claims include: "3 grams containing more fiber than 1 slice of Brand X bread", and "33% less sodium per x serving than our regular potato chips". The conditions for using such claims are also specified in Canada's nutrient content claims for specific nutrients, such as energy and calorie claims.
(2) Health claims, which are statements that describe the potential health effects of a food product when consumed as part of a healthy diet. Canada has different types of health claims, all of which highlight the link between a food or a constituent of a food and health. There are three types of health claims on foods:
l General health claims refer to broad claims that promote health through healthy diet or that provide dietary guidance, but do not refer to a specific or general health effect, disease, or health condition. Typical permitted terms include "nutritious" and "healthy".
l Function claims are statements about the specific benefits a food has on normal body functions. Table of Acceptable Food or Food Constituent Function Claims shows the acceptable claims and corresponding use conditions. Currently, only three foods or food constituents are permitted to use such claims. They are coarse wheat bran, green tea, and psyllium. Function claims contain two subcategories of claims:
Nutrient function claims are claims describing the well-established roles of energy or nutrients that are essential for the maintenance of good health or normal growth and development. Acceptable Nutrient Function Claims Table lists two general nutrient function claims for all nutrients and specific nutrient function claims for 28 nutrients. Take protein for an example, it's acceptable to use three protein-related claims, namely, "helps build and repair body issues", "helps build antibodies", and "helps build strong muscles". Notably, whenever a nutrient function claim is made, the consumer must be imformed as to the amount of energy or nutrient present in a serving of the food, which can be done through a declaration in the Nutrition Facts table (NFT) or a quantitative statement outside the NFT.
Probiotic claims are claims about the health benefits or effects of probiotics. Table of Acceptable Non-Strain Specific Claims for Probiotics includes 16 eligible bacterial species and 4 acceptable claims such as "probiotic that naturally forms part of the gut flora". The quantity of probiotic microorganisms contained in the product at the end of its shelf life should be declared in colony forming units (cfu) per serving.
l Disease risk reduction claims, which are statements that link a food to a lower risk of developing a disease or condition, such as "a healthy diet rich in the variety of vegetables and fruit may help reduce the risk of some types of cancer". Table of Disease Risk Reduction Claims lists the permitted disease risk reduction claims, as well as the conditions for food, food label and advertisement to meet.
Therapeutic claims are a type of disease risk reduction claims. They link a food to the treatment or improvement of a disease, condition or body function. For example, "Oat fibre helps lower cholesterol".
Products classified as health food in China are defined as natural health product (NHP) in Canada. More importantly, NHP is regulated as a subset of drugs in Canada, therefore, the claims for NHP does not belong to food claims.
In general, nutrient content claims and health claims can be used voluntarily on any food that meets the criteria established by Health Canada. However, some health claims, such as disease risk reduction claims and therapeutic claims, must be submitted to Health Canada for approval before use.
Comparison and conclusion
The regulatory systems for food claims in China and Canada have both similarities and differences. Some of the main points of comparison are:
l Both countries have a dual-track system for regulating food claims. Both systems distinguish between nutrition claims and health (function) claims, with varying requirements for evidence and approval.
l Both countries allow the use of (health) function claims. However, China restricts such claims to be only used for health food products, while Canada permits their use in common food products.
l Compared to China, Canada's legislations encompass a broader range of claims for food products.
l China strictly prohibits food products from having claims related to disease prevention and treatment. In contrast, Canada conditionally allows therapeutic claims for food products.
In conclusion, China and Canada have distinct regulatory systems for food claims. These differences may pose some challenges and opportunities for the food industry and trade between the two countries. Food manufacturers or exporters should have a comprehensive understanding of these similarities and differences, which will definitely facilitate the food trade and benefit the consumers.
Source: Chemlinked
Note: This article is compiled by Antion. Please indicate the source for reprint.