On October 18, 2023, National Health Commission (NHC) announced to seek public comments on 11 national food safety standards, involving dairy products, food contact materials (FCM), food for special medical purposes (FSMP), canned food, and food additives. Any comments can be submitted online prior to December 15, 2023.
On October 17, 2023, China Food Additives and Ingredients Association (CFAA) released 13 food safety national standard exposure drafts for public comments. The drafts include 9 emulsifier GB standards and 4 sweetener standards. Any comments can be submitted before November 17, 2023.
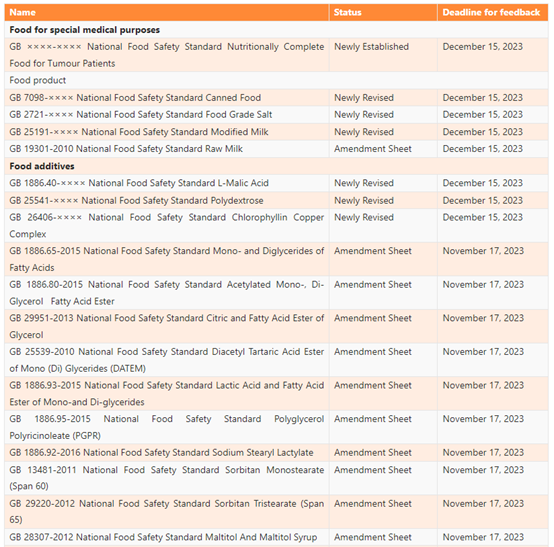
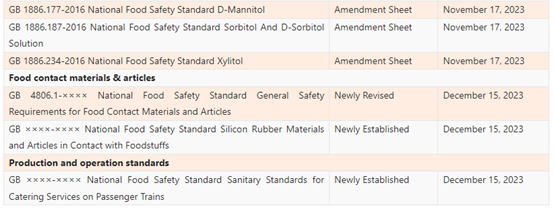
List of the Proposed GB Standards
Key Changes in Select Standards
Dairy product
GB 25191-×××× Modified Milk
Seeing the application potential of concentrated dairy products like condensed milk and evaporated milk, the competent authority proposes to include the concentrated dairy products as the raw materials of modified milk. Hence, the product definition is revised. Concentrated dairy products used as ingredient should comply with GB 13102 National Food Safety Standard Condensed Dairy Products.
In addition, the draft allows a small quantity of floating fat in modified milk. With the development of breeding capabilities, the fat content in sterilized milk increases nowadays. The fat floating phenomenon that occurs during storage is an inherent characteristic of the dairy product and does not pose any safety hazards.
In terms of labeling, "xx milk" can be used as the product name of modified milk according to the product characteristics, with "modified milk" or "xx modified milk" being indicated as the product category. This requirement is actually sourced from the NHFPC announcement issued in 2014. Additionally, enterprises can use the words like skimmed, partial-skimmed and full to describe the fat content in the product name.
GB 19301-2010 Raw Milk No.1 Amendment Sheet
The Amendment Sheet revises the acidity level from 12-18 to 10-18, and removes the application scope of Holstein Cow, which means the limitation for this indicator applies to all dairy cattle species.
Food for special medical purposes
GB ××××-×××× Nutritionally Complete Food for Tumour Patients
In China, FSMP products for people aged above 1 year are classified into nutritionally complete foods, specific nutritionally complete foods and nutritionally incomplete foods. Products under this new draft standard are specific nutritionally complete foods. The contents of this draft include scope, terms and definitions, technical requirements, packaging, and labeling requirements.
Key technical requirements:
The draft requires that the energy density of nutritionally complete food for tumour patients should not be less than 1.2 kcal/ml(g). The protein content of the product should not be lower than 0.96g/100kJ (4.0g/100kcal), and the protein must come from high-quality sources, including whole proteins, hydrolysed proteins, and peptides.
Additionally, optional nutrients include arginine, glutamine, leucine, β-hydroxy-β-methylbutyrate calcium, taurine, L-carnitine, nucleotides, dietary fibre, etc.
Labeling:
As per the draft, the product label should include a description of the product's formulation, nutritional features, protein source and proportion. It should also indicate the product category, intended user population, preparation instructions, product's osmolarity, and recommended storage duration. In addition, the following statements should be labeled as well on the package:
l "Not suitable for individuals other than the target population."
l "Please use under the guidance of a doctor or clinical nutritionist."
l "Not for use in parenteral nutrition support or intravenous injection."
l "Regular monitoring of nutrient deficiencies is recommended."
Food additives
Amendment Lists for 4 sweeteners and 9 emulsifiers consulted by China Food Additive and Auxiliary Materials Association
In practical commercial production, manufacturers need to add anti-caking agent, antioxidant, acidity regulator, etc. to maintain the normal function and stability of these sweeteners and emulsifiers. However, such permission is not mentioned in the current standards. Moreover, the addition of these substances will lead to the non-compliance with the limitation of some current indicators especially the ignition residue limits. Therefore, the competent authority proposes to clarify the usage permission and states that food additive products for commercial use are exempted from the limitation requirements of such indicators.
GB standards for 3 food additives consulted by NHC
The proposed amendments for L-malicacid, polydextrose and chlorophyllin copper complex mainly cover physicochemical indicators and testing methods.
Source: NHC and Chemlinked
Note: This article is compiled by Antion. Please indicate the source for reprint.