On December 3, 2023, State Administration for Market Regulation (SAMR) unveiled the updated Administrative Measures for Registration of Foods for Special Medical Purpose, replacing the 2016 version. Major revisions include priority review and approval procedures, situations not approved for registration, labels, penalties, and measures to optimize the registration procedure. The new version will come into force on January 1, 2024.
Major Revisions
The revised Measures is established with the objective of promoting FSMP innovation as well as expediting the review and approval process, centering on clinical nutrition needs.
Priority review and approval procedures
The authority establishes priority review and approval procedures. Applicants can apply for priority review in either of the following circumstances.

The applicant should communicate with Center for Food Evaluation (CFE) under SAMR beforehand and, upon confirmation, simultaneously submit a priority review application along with the registration application. The technical evaluation period is shortened to 30 working days from 60 working days. In addition, the on-site inspections and sample testing is prioritized.
Situations not allowed for registration
The updated version clearly defines 7 situations that are not approved for registration. Details are shown in the table below.
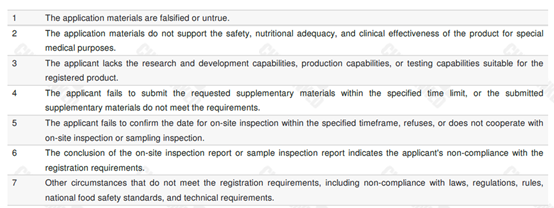
Situations applicable for application withdrawal
The revised Measures clarifies the circumstances where application can be withdrawn. The applicant may request to withdraw the application during the evaluation process. However, if any illegal activities are discovered during the technical evaluation, on-site inspection, or sampling inspection, such as concealing true information or providing false information, the registration application cannot be withdrawn.
Requirements adjusted for applicants
The revised Measures further emphasizes the capabilities and legal responsibilities of the applicants.
1. The revised Measures clarifies that applicants are responsible for the authenticity, completeness, legality, and traceability of the submitted materials, and shall bear legal responsibilities. Applicants should cooperate with CFE in conducting on-site inspections, sampling inspections, and other registration-related activities, while providing necessary working conditions for these inspections and activities.
2. The Measures introduces the requirement of submitting the applicant's qualifications documents to the CFE, together with FSMP application.
Changes in registration alteration and extension requirements
Registration alteration: The updated Measures introduces the requirement of submitting a product alteration justification report for registration alteration. The registration alteration is not required if the following conditions are met: the variety of food ingredients and food additives remains unchanged, the sequence of ingredients remains unchanged, and the nutritional composition table remains unchanged. Additionally, reasonable fluctuations or adjustments within a certain range of the ingredient quantities are allowed.
Registration extension: To extend registration, it is necessary to submit materials related to the company's research and development capabilities, production capabilities, and testing capabilities, as well as the tracking evaluation on product safety, nutritional adequacy, and clinical effectiveness of special medical purposes. Additionally, applicant is required to obtain an opinion letter for registration extension from the provincial level food safety administrative department. Failure to maintain research and development capabilities, production capabilities, or testing capabilities will result in the denial of registration extension for the applicant.
Revised contents of FSMP registration certificate
The revised Measures requires the applicants to provide product technical requirements in the annex of the registration certificate, aiming to ensure product quality and safety as well as strengthen the technical evaluation during registration.
l In addition to compliance with relevant national food safety standards, companies should organize production in accordance with the product technical requirements specified in the registration certificate.
l The on-site inspection samples collected dynamically should be tested in accordance with the national food safety standards and the product technical requirements.
Revisions in optimizing the registration procedure
Clarified requirements for on-site inspection: The revised Measures provides clear guidelines for CFE to organize on-site inspections and sampling inspections on applicants' production sites, as well as on-site inspections of clinical trials based on food safety risks. When necessary, inspection of food raw material lists or food additive lists may be conducted on relevant manufacturers.
Reduced duration for clinical trial inspections: The duration for conducting on-site inspections on clinical trials has been shortened from 40 working days to 30 working days.
Legal Validity of Electronic Certificates: Electronic certificates hold the same legal validity as their paper counterparts. CFE can inform applicants about inspection matters through written or electronic means
Updated labeling requirements
The main display area of the label should clearly indicate the product name, registration number, intended users, and the statement "Please use under the guidance of a doctor or clinical nutritionist."
It is required to describe the formulation characteristics or nutritional features of the product, which reflect the attributes of the FSMP product.
No functional claims should be made regarding the nutrients and other ingredients in the product to avoid misleading consumers.
Changes to the punishments
The updated version introduces stricter punishments for illegal activities that harmful consequences. For instance, the maximum fine for applicants who obtain registration certificates through deception, bribery, or other improper means result in harmful consequences increases from 30,000 to 200,000 yuan. The same penalty also applies to applicants who engage in the forgery, alteration, resale, rental, lending, or transfer of FSMP registration certificates that lead to harmful consequences.
The punishment for minor violations is reduced. Applicants who fail to apply for changes in matters that do not affect the safety, nutritional adequacy, and clinical efficacy of special medical purposes of the product shall face a fine ranging from 1,000 to 10,000 Yuan instead of the previous range of 10,000 to 30,000 Yuan. In addition, they shall be ordered to make corrections within a specified time limit.
Source: SAMR and Chemlinked
Note: This article is compiled by Antion. Please indicate the source for reprint.