The NHC issued the National Food Safety Standards Infants Formula (Draft for Comments), National Food Safety Standards Older Infants Formula (Draft for Comments) and National Food Safety Standards Young Children Formula (Draft for Comments) in 2018. After more than 2 years, on March 18, 2021, the NHC issued 50 new national food safety standards, including: National Food Safety Standards Infant Formula (GB 10765-2021), National Food Safety Standards for Older Infants Formula (GB 10766-2021) and National Food Safety Standards for Young Children (GB 10767-2021) (hereinafter referred to as the New Standards), which will be implemented on February 22, 2023. The government encourages companies to implement New Standards before they are implemented. Antion analyzes and interprets the main contents as follows.
From the overall revision, New Standards put forward more stringent requirements for product quality requirements. Nutrient indicators have been adjusted. New Standards sets maximum values for all nutrients of older infants formula. And refine product standards and split the current 2 standards into 3 standards to enhance the scientific nature.
Modify terms and definitions
The National Food Safety Standard Older Infants Formula (GB 10766-2021) clearly divides older infant formula into dairy-based older infants formula and soy-based older infants formula, and emphasizes that the main protein source requirements, protein sources of dairy-based and soy-based product must not be mixed.
Adjust protein indicators
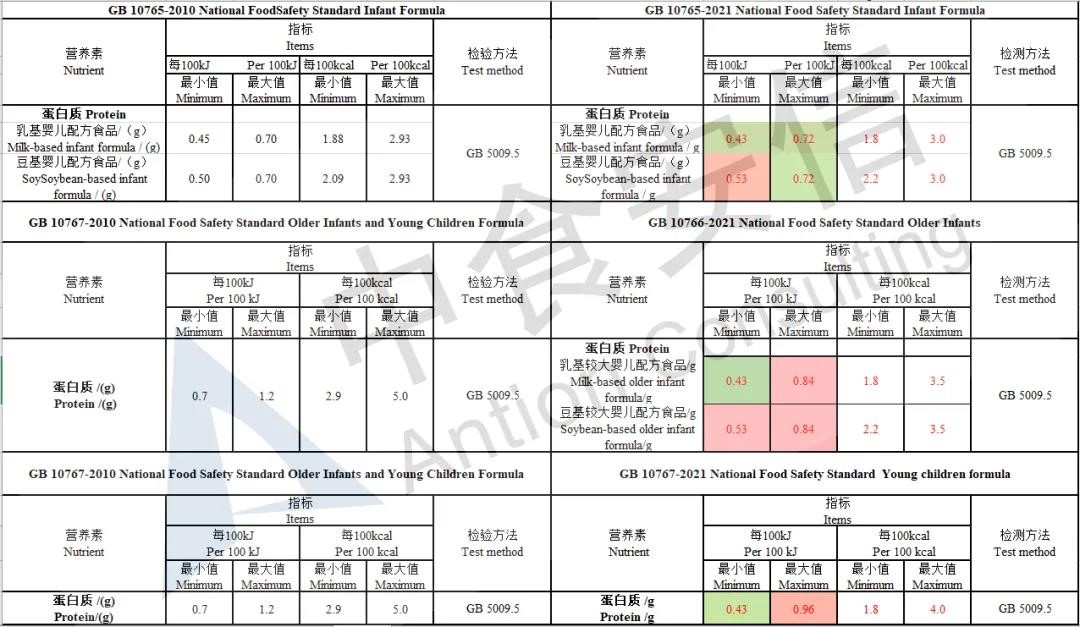
Add the requirements of proportion of whey protein for older infants formula, the content of whey protein in milk-based older infants formula should be ≥ 40%.
Adjust carbohydrate indicators
The carbohydrate indicators of infant formula are fine-tuned, new requirement of not to use sucrose as a source of carbohydrates is added, new indicators of carbohydrate-related requirements for older infants formula and young children formula are added. Among them, older infants formula shall not use fructose and sucrose as a source of carbohydrates, the ratio of lactose for older infants formula shall be ≥ 90%, and for young children formula (except lactose-free and low-lactose products) shall be ≥ 50%.
Improve the vitamin and mineral indicators
For infants formula, the vitamin and mineral indicators are adjusted. Among them, the adjustment of vitamin D and niacin is larger, the indicator of vitamin D is adjusted from “0.25-0.6μg per 100kJ” to “0.48-1.2μg per 100kJ”, the indicator of niacin is adjusted from “70-360μg per 100kJ” to “96-359μg per 100kJ”.
Increase maximum indicators of vitamin for older infants formula and young children formula. New Standards adjusts the essential ingredients. Choline is adjusted from optional ingredients to essential ingredients for infant formula and older infants formula, while increasing the content requirements. For older infants formula, manganese and selenium are adjusted from optional ingredients to essential ingredients, the maximum level of magnesium, calcium, phosphorus and iodine are added. For young children formula, the maximum level of magnesium, calcium, phosphorus and iodine are added.
The landing of the New Standards will have a significant impact on infant formula powder producers, who are facing the situation of formula re-registration. Antion recommends that infant formula powder enterprises should assess the existing product formulas as soon as possible, and advance planning to prepare for re-registration.
If you want to know more about New Standards and infant formula registration requirements, please contact us!
Lillian Fan
Tel: 010-51301566
email: Lillian.fan@antion.net
Relevant Reading
Introduction of Food Related Application & Registration Services
2020 Industry Report for Infant Formula Milk Powder (Excerpt)